We created a new material from orange peel that can clean up mercury pollution

Mercury pollution is one of the most insidious problems in our environment. Today my colleagues and I at Flinders University have unveiled a new material than can scrub mercury from the environment, as a result of research to be published this week. The material – sulphur-limonene polysulphide – binds to mercury and changes colour, helping us see how effective it is.
Mercury is a neurotoxin. Developing fetuses are most vulnerable and mercury poisoning can cause developmental delays in unborn babies.
The primary pathway for mercury in people is through eating fish. Mercury accumulates in animals’ tissues, so fish at the top of the food chain can contain high and potentially toxic levels. It can cause both chronic and acute effects in marine life. Pregnant women are recommended to avoid eating large amounts of certain types of fish.
Since the Industrial Revolution humans have increased the concentration of mercury in the ocean by 10%, and the rate is increasing.
The major sources of mercury in water in Australia are from water supply, manufacturing, mining, oil and gas extraction, and electricity generation.
Our new material not only removes mercury from water and soil, but is created from industrial waste products. So our material effectively solves two problems: cleaning up pollution, and doing it sustainably.
When life gives you limonene
Sulphur-limonene polysulphide is a polymer or large molecule made, as the name suggests, from sulphur and limonene. Sulphur is the element known for smelling like rotten eggs when combined with hydrogen to produce hydrogen sulphide. Limonene is found in the oil of orange peel and other citrus fruits.
Both are waste products. The petroleum industry produces between 60 million and 70 million tonnes of sulphur each year. There are literally mountains of sulphur lying around the globe, unused.
The citrus industry produces more than 70 thousand tonnes of limonene each year. Finding a use for these materials is an important contribution to the preparation of sustainable materials.
The vast majority of polymers (plastics, rubber, paints, coatings etc) are derived from finite supplies of petroleum. Identifying new sources is therefore critical for the sustainable production of polymers.
In waste-valorisation, new uses are found for byproducts that are otherwise stockpiled as waste. Contributing to this goal, the new polymer in this research is made entirely from industrial byproducts sulphur and limonene – no other parts are required.
Cleaning up mercury
At the outset of the project, we were primarily interested in making a new polymer from widely available and sustainable materials. There have been some recent reports on using sulphur and limonene as starting materials for very different types of polymers. We simply wanted to see if we could use both sulphur and limonene in the same polymer.
The chemical merger of these two industrial byproducts proved remarkably easy. The real surprise came when we studied its behaviour in metal binding. Because the polymer has a high sulphur content, we anticipated it should have a high affinity for metals that bond to sulphur. This was indeed the case.
Moreover, we found it could remove more than 50% of the mercury from water after only a single treatment. Subsequent treatments can be used to approach mercury levels suitable for drinking.
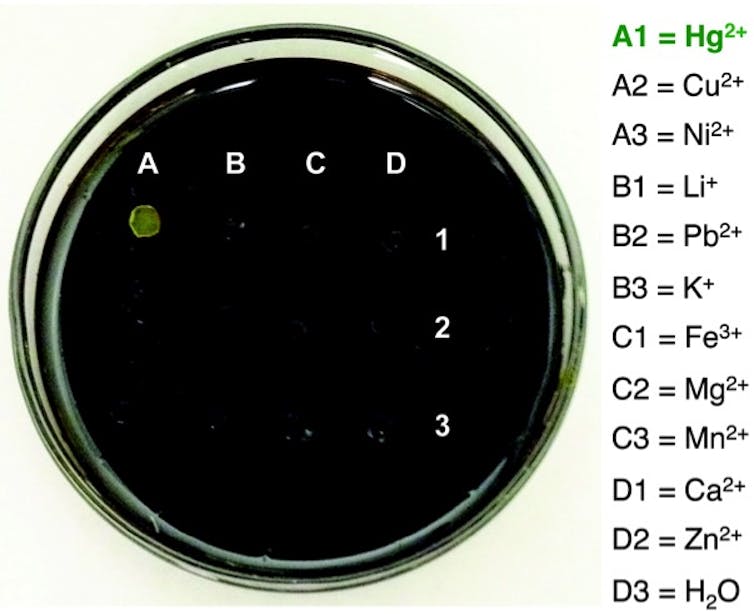
While there are other materials that are very efficient at removing mercury from water, our material is unique in that it is far less expensive. Also, when the polysulphide is exposed to mercury, it changes colour. This colour-changing or chromogenic response was a very welcome surprise. We can use this property as a sensor for mercury.
Our preliminary studies indicate that the sulphur-limonene polysulphide is not toxic. This is a critical finding if the polymer is to be used directly in natural ecosystems such as rivers, lakes and oceans.
We are currently exploring commercialisation of the technology. These efforts are aimed at partnering with existing industries and environmental agencies to produce and use the material in large-scale remediation efforts. We are also weighing options for seeking investment for a startup company.
Using the material in toxic waste clean-up may be a year or more away, but we are pursuing these efforts actively with a partnership between Flinders Partners (the commercialisation arm of Flinders University) and The University of Tulsa (collaborators in this research).
We aim to use this material to remove mercury from groundwater and soil. We are also exploring its use as a component in water filters to ensure safe drinking water. More generally, we hope to inspire other scientists and engineers to develop novel and useful materials that address urgent challenges in sustainability.
Justin Chalker declares competing financial interests. He is an inventor on a patent application for this technology (PCT/US15/55205). Justin Chalker also receives funding from the Australian Research Council (DE150101863).